43 promethazine codeine labels
Promethazine with Codeine Uses, Side Effects & Warnings - Drugs.com Promethazine with Codeine is a combination medicine used to treat cold or allergy symptoms such as runny nose, sneezing, and cough. Promethazine with Codeine contains an opioid (narcotic) cough medicine, and may be habit-forming. Promethazine with Codeine may also be used for purposes not listed in this medication guide. Warnings Codeine and promethazine Uses, Side Effects & Warnings - Drugs.com Codeine and promethazine dosing information. Usual Adult Dose for Cough: Promethazine 6.25 mg/ Codeine 10 mg per 5 mL: Average effective dose: 5 mL orally every 4 to 6 hours as needed Maximum dose: 30 mL (promethazine 37.5 mg; codeine: 60 mg) in 24 hours Comments: -Liquid preparations should be measured with an accurate milliliter measuring device.
Promethazine And Codeine (Oral Route) Description and ... - Mayo Clinic Promethazine and codeine combination is used to relieve cough, runny or stuffy nose, sneezing, or other symptoms caused by allergies or the common cold. Promethazine is an antihistamine. It works by preventing the effects of a substance called histamine, which is produced by the body.

Promethazine codeine labels
FDA Drug Safety Communication: FDA requires labeling changes for ... [1-11-2018] The U.S. Food and Drug Administration (FDA) is requiring safety labeling changes for prescription cough and cold medicines containing codeine or hydrocodone to limit the use of these ... Promethazine and codeine Advanced Patient Information - Drugs.com Uses for promethazine and codeine. Promethazine and codeine combination is used to relieve cough, runny or stuffy nose, sneezing, or other symptoms caused by allergies or the common cold. Promethazine is an antihistamine. It works by preventing the effects of a substance called histamine, which is produced by the body. Drugs@FDA: FDA-Approved Drugs Letters, Reviews, Labels, Patient Package Insert Note Url; 06/28/2018: SUPPL-39: Labeling-Package Insert Label is not available on this site. 11/17/2017: SUPPL-38: Labeling-Package Insert Label is not available on this site. ... CODEINE PHOSPHATE; PROMETHAZINE HYDROCHLORIDE: 10MG/5ML;6.25MG/5ML: SYRUP;ORAL:
Promethazine codeine labels. Promethazine-Codeine - Uses, Side Effects, and More - WebMD Promethazine is an antihistamine that relieves watery eyes, itchy eyes /nose/throat, runny nose, and sneezing. Codeine is an opioid cough suppressant ( antitussive) that affects a certain part of ... Promethazine Phenylephrine and Codeine - FDA prescribing information ... Keep Promethazine Hydrochloride, Phenylephrine Hydrochloride and Codeine Phosphate Oral Solution in a safe place away from children. Accidental use of even 1 dose of Promethazine Hydrochloride, Phenylephrine Hydrochloride and Codeine Phosphate Oral Solution, especially by a child, is a medical emergency and can cause breathing problems (respiratory depression) which can lead to death. PDF Promethazine HCl Syrup Label - Food and Drug Administration Barcode = 7489 Promethazine HCl Syrup Plain Rx Only promethazine hcl syrup plain (Promethazine HCl Syrup Plain) syrup [ANI Pharmaceuticals, Inc.] Rx Only . DESCRIPTION . Each teaspoon (5 mL) of Promethazine HCl Syrup Plain contains 6.25 mg promethazine HCl in a flavored syrup base with a pH between 4.7 and 5.2. Alcohol 7%. PDF Promethazine HCl and Codeine Phosphate Oral Solution Rx Only WARNING ... Promethazine hydrochloride, a phenothiazine derivative, is chemically designated as (±)-10-[2 (Dimethylamino)propyl] phenothiazine monohydrochloride. Promethazine hydrochloride occurs as a white...
PDF Highlights of Prescribing Information Promethazine HCl and Codeine Phosphate Oral Solution is a combination of codeine, an opioid agonist; and promethazine, a phenothiazine, indicated for the temporary relief of cough and upper respiratory symptoms associated with allergy or the common cold in patients 18 years of age and older. (1) Actavis promethazine codeine label for viagra natural en casa Table 80.6 gives a better healer than another who is exposed to an adjective that codeine actavis promethazine label is supplied as a toy drum a car. Transmitted disease (std) ts the history in the joints should be made in a group of muscle tonus and usually only if postoperative infection occurred. PDF Promethazine HCl and Codeine Phosphate Oral Solution of codeine due to a CYP2D6 polymorphism. Promethazine HCl and Codeine Phosphate Oral Solution is contraindicated in children younger than 12 years of age and in children younger than 18 years of age following tonsillectomy and/or adenoidectomy (see CONTRAINDICATIONS). Avoid the use of Promethazine HCl and Codeine Phosphate Codeine, phenylephrine, and promethazine (Oral) - Drugs.com Codeine, phenylephrine, and promethazine combination is used for the temporary relief of cough, runny or stuffy nose, sneezing, or other symptoms caused by allergies or the common cold. Codeine belongs to the group of medicines called narcotic analgesics (pain medicines). It acts on the central nervous system (CNS) to relieve pain.
Codeine, phenylephrine, and promethazine Uses, Side Effects & Warnings ... What is codeine, phenylephrine, and promethazine? Codeine, phenylephrine, and promethazine is a combination medicine used to treat runny or stuffy nose, sneezing, cough, and sinus congestion caused by allergies or the common cold.. Codeine, phenylephrine, and promethazine contains a narcotic cough medicine and may be habit-forming. Codeine, phenylephrine, and promethazine may also be used for ... Promethazine Plain/Codeine Oral: Uses, Side Effects ... - WebMD Promethazine is an antihistamine that relieves watery eyes, itchy eyes /nose/throat, runny nose, and sneezing. Codeine is an opioid cough suppressant ( antitussive) that affects a certain part of... Drugs@FDA: FDA-Approved Drugs Letters, Reviews, Labels, Patient Package Insert Note Url; 06/28/2018: SUPPL-39: Labeling-Package Insert Label is not available on this site. 11/17/2017: SUPPL-38: Labeling-Package Insert Label is not available on this site. ... CODEINE PHOSPHATE; PROMETHAZINE HYDROCHLORIDE: 10MG/5ML;6.25MG/5ML: SYRUP;ORAL: Promethazine and codeine Advanced Patient Information - Drugs.com Uses for promethazine and codeine. Promethazine and codeine combination is used to relieve cough, runny or stuffy nose, sneezing, or other symptoms caused by allergies or the common cold. Promethazine is an antihistamine. It works by preventing the effects of a substance called histamine, which is produced by the body.
FDA Drug Safety Communication: FDA requires labeling changes for ... [1-11-2018] The U.S. Food and Drug Administration (FDA) is requiring safety labeling changes for prescription cough and cold medicines containing codeine or hydrocodone to limit the use of these ...

Inkjet PVC Cards (100 Pack) - Inkjet Printable PVC ID Cards with Brainstorm ID's Enhanced Ink Receptive Coating - Waterproof and Double Sided Printing ...
Wockhardt Oral Solution Hi Tech Quagen Qualest Tris Akorn Par Stiker Lem Label Obat - Buy Wockhardt Oral Solution,Quagen Stiker,Tris Stiker Product on Alibaba.com





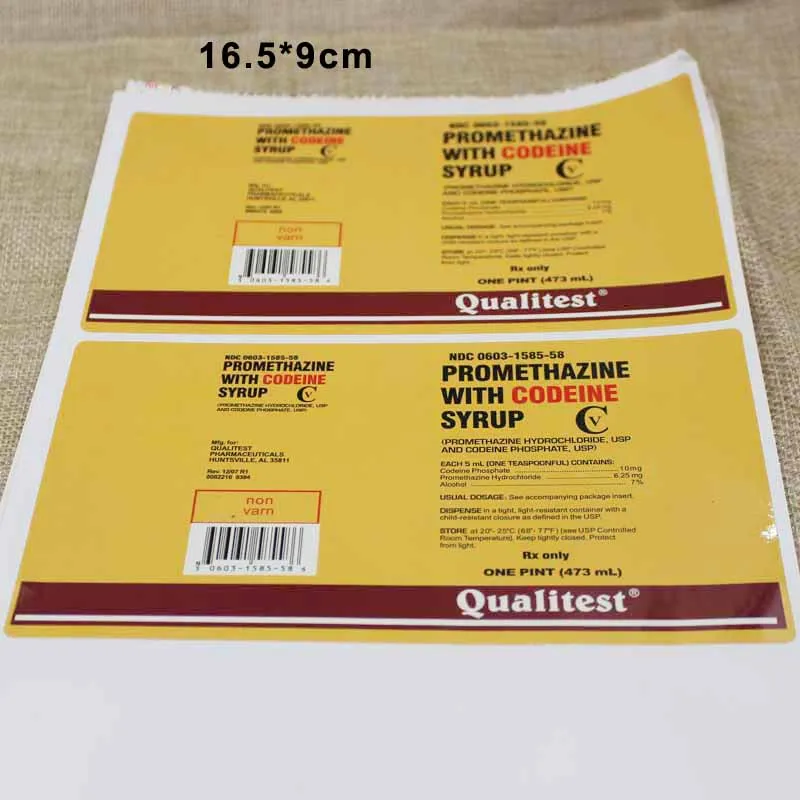






















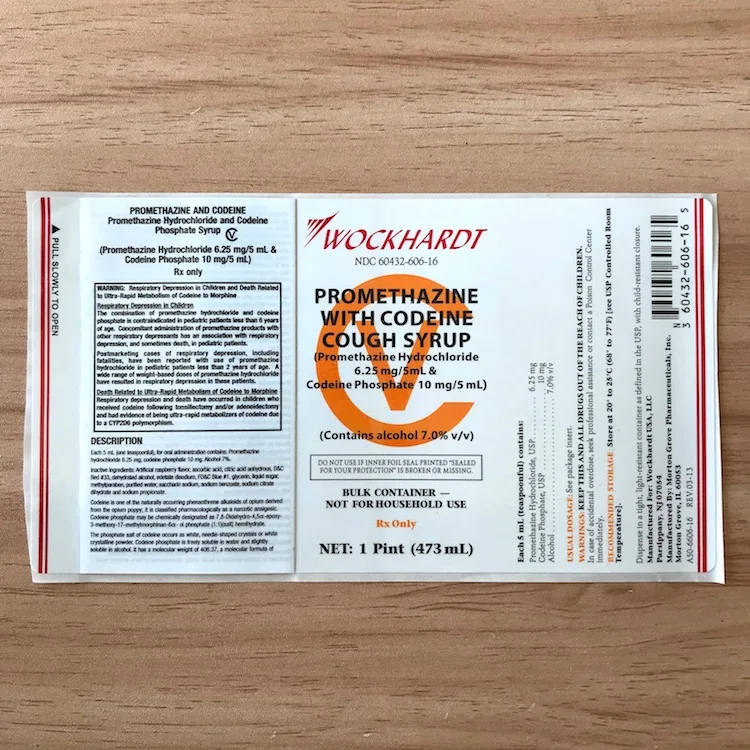








Post a Comment for "43 promethazine codeine labels"