42 label the following reaction coordinate diagram.
Label The Following Reaction Coordinate Diagram. - Chapter 1 ... Label the following reaction coordinate diagram by matching between letters and numbers: A graph is shown with the label, "reaction coordinate," on the x figure 1. Label the following reaction coordinate diagram enthalpy of reaction activation energy (forward) reactant (s) transition state = 0 . d-Metal Complexes - uml.edu In this case, the d z 2 orbital drops even lower in energy, and the molecule has the following orbital splitting diagram. As a result of these distortions, there is a net lowering of energy (an increase in the ligand field stabilization energy) for complexes in which the metal has a d 7 , d 8 , or d 9 configurations, and thus electrons would occupy the upper e g set if an octahedral …
How can I draw activation energy in a diagram? | Socratic You follow a series of steps. > 1. Draw and label a pair of axes. Label the vertical axis "Potential Energy" and the horizontal axis "Reaction Coordinate". 2. Draw and label two short horizontal lines to mark the energies of the reactants and products. 3. Draw the energy level diagram. There must be a hump in the curve to represent the energy level of the activated complex. 4. Draw and label ...
Label the following reaction coordinate diagram.
Answered: Answer the following questions… | bartleby This reaction coordinate diagram corresponds to a mechanism with Energy steps. Answer the following questions regarding the reaction coordinate diagram below. m Reaction Coordinate a. This reaction coordinate diagram is consistent with which mechanism (E1 or E2)? b. Reaction Coordinate Diagrams The diagram below is called a reaction coordinate diagram. It shows how the energy of the system changes during a chemical reaction. In this example, B is at a lower total energy than A. This is an exothermic reaction (heat is given off) and should be favorable from an energy standpoint. The energy difference between A and B is E in the diagram. Label The Energy Diagram (7 Bins) And Indicate Which Reaction ... Answer to Label the energy diagram (7 bins) and indicate which reaction corresponds to the energy diagram. The y-axis of the Maxwell-Boltzmann graph can be thought of as giving the number of molecules per unit speed. So, if the graph is higher in a given region. If playback doesn't begin shortly, try restarting your device.
Label the following reaction coordinate diagram.. PDF 1. (18 points) making ethane thiolate: Derive the rate law for the ... Draw a new reaction coordinate diagram on the axes above ... Draw a More O'Ferrall-Jencks plot for the following reaction on the diagram below to be analogous to SN1 and SN2 on a carbon. Label the axes and corners. (4 points) b) If the BDE of a Si-Cl bond is 90 kcal/mol, while that of Si-O is 110 kcal/mol, place a dot along the ... (PDF) Engineering Chemistry by Jain & Jain - Academia.edu Enter the email address you signed up with and we'll email you a reset link. 5.2 Method of Joints – Engineering Mechanics: Statics Label each force in the diagram. Include any known magnitudes and directions and provide variable names for each unknown. Write out the equilibrium equations for each of the joints. You should treat the joints as particles, so there will be force equations but no moment equations. This should give you a large number of equations. Solved Label the following reaction coordinate diagram. - Chegg Question: Label the following reaction coordinate diagram. Energy Reactant (s) Transition State Product (s) Activation Energy (forward) Transition State Activation Energy (forward) Energy Enthalpy of Enthalpy of Reaction Product (s) Reaction AHrxn Reactant (s) Reaction Coordinate Reaction Coordinate Reset Zoom This problem has been solved!
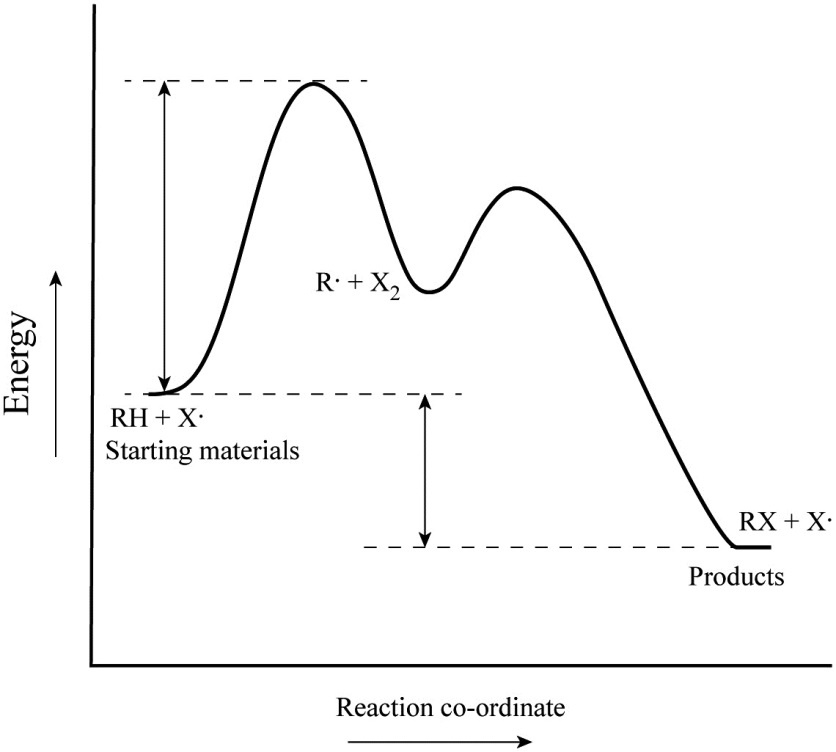
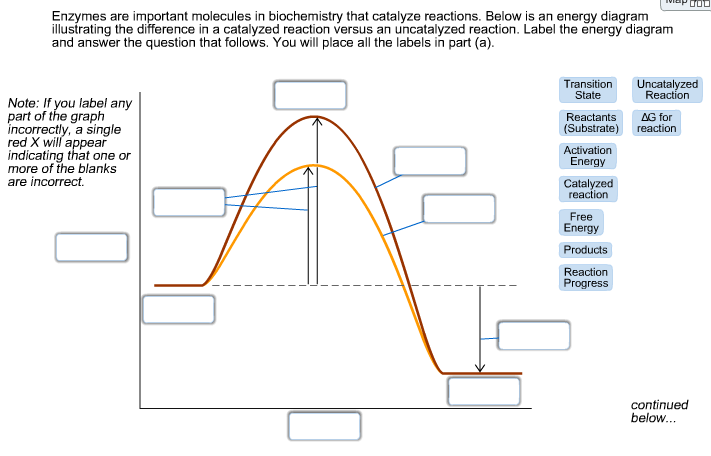
14.9: The Effect of Temperature on Reaction Rates 20.08.2020 · Figure \(\PageIndex{4}\) shows both the kinetic energy distributions and a potential energy diagram for a reaction. The ... measurements of the rate of tree cricket chirping (\(f\)) as a function of temperature (\(T\)). Use the data in the following table, along with the graph of ln[chirping rate] versus \(1/T\) to calculate \(E_a\) for the biochemical reaction that controls … chem (kinetics pt 2) Flashcards | Quizlet Label the following reaction coordinate diagram by matching between letters and numbers: (diagram in kinetics pt 2 folder in energy diagram folder on desktop) 1- J 2- F 3- A ... -Label the multi-step reaction energy diagram below using the letters corresponding to the labels on the left. There are more labels than needed; each label can be used ... 6.4.1: Eyring equation - Chemistry LibreTexts 23.09.2021 · Once the energy barrier is overcome, the reaction is able to proceed and product formation occurs. Figure \(\PageIndex{1}\): Reaction coordinate diagram for the bimolecular nucleophilic substitution (\(S_N2\)) reaction between bromomethane and the hydroxide anion. form Wikipedia. The rate of a reaction is equal to the number of activated ... Chapter 7 Flashcards | Quizlet On this graph, the x-axis is the reaction coordinate, while the y-axis is energy. Adding a catalyst to a reaction can stabilize the transition state, thereby reducing the activation energy of that reaction. However, the overall free energy of the reaction remains the same with or without a catalyst. Label the following figure.
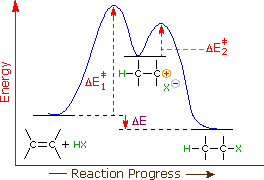
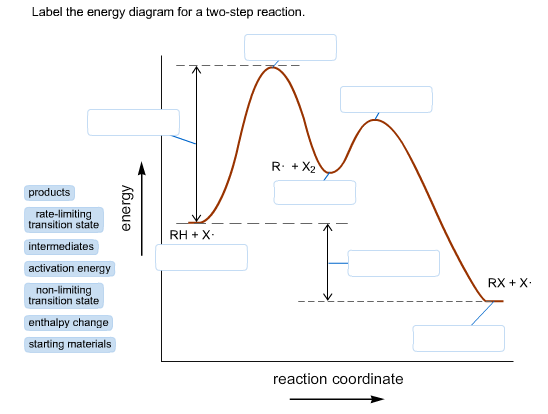
Draw a reaction coordinate diagram for a two-step reaction i | Quizlet Explanations Question Draw a reaction coordinate diagram for a two-step reaction in which the first step is endergonic, the second step is exergonic, and the overall reaction is endergonic. Label the reactants, products, intermediates, and transition states. Explanation Verified Reveal next step Reveal all steps Arrhenius Theory and Reaction Coordinates - Chemistry 302 The reaction above has three steps (three barriers) and two intermediates. On the far left of the diagram are the reactant species and on the far right are the product species. Transition State The transition state is the high energy point between two minima along the reaction coordinate. Each step in a mechanism will have a transition state. The Reaction Coordinate Diagram Questions - Chem Homework Help The Reaction Coordinate Diagram Questions Homework 4: Chapter 6 Part 1 5 points Consider the Reaction Coordinate Diagram and answer the questions. How many transition states are in this reaction? 1 2 3 4 2. How many intermediates are in this reaction? 1 2 3 4 3. How many steps are in this reaction? 1 2 3 4 4. Answered: Reaction coordinate diagrams: Two-step… | bartleby Transcribed Image Text: Reaction coordinate diagrams: Two-step diagram. Label the reactant (R), product (P), intermediate (I"), and transition states (TS; and TS2), plus the axis with appropriate units/labels. 1) Break the diagram up into 2 steps. Which one is the slow step? The fast step? 2) Endothermic or exothermic? Suppose a is added to ...
Label The Following Reaction Coordinate Diagram / Consider The Energy ... Label the following reaction coordinate diagram enthalpy of reaction activation energy (forward) reactant (s) transition state = 0 . If each interval on the axis labeled potential. The diagram below is called . Energy reactant (s) transition state product (s) activation energy (forward) . Is the forward reaction endothermic or exothermic?
Admissions - Winston-Salem State University WSSU is a vibrant student-centered learning community, embracing educational excellence in a caring culture that supports student success. Prepare for today's in-demand jobs with innovative programs in more than 40 majors. Ignite your passion, discover your strength, and prepare to make a difference in the world.
SOLVED:Draw reaction coordinate diagram for slow, concerted endothermic ... Label axes, reactant; product; transition state; intermediate, activation energy and AG Draw reaction coordinate diagram for fast, stepwise reaction exothermic reaction: Label axes, reactant, product; transition state, intermediate_ activation energy and 4G If there Is concerted reaction between the following chemicals, write the rate Iaw for ...
Free Body Diagram - Definition, Examples, Solved Problems, FAQs Normally, a free body diagram consists of the following components: A simplified version of the body (most commonly a box) ... Draw a coordinate system and label positive directions. 4. Draw the contact forces on the dot with an arrow pointing away from the dot. The arrow lengths should be relatively proportional to each other. Label all forces. 5. Draw and label our long-range …
Answered: Draw a reaction coordinate for the… | bartleby The three step mechanism for the following reaction can be seen below… Q: Fill in the intermediate step (s) in the [] and the product (s) in the boxes. H2SO, / H20 1.BH3:THF… Q: Consider the reaction: CH3CH2· + Br2 → CH3CH2B1 + Br . Given that this reaction has an activation… Q: e intermediate formed in the first step of the mechanism. Q: 16.
The following diagram shows a reaction profile. Label the components ... This problem shows a picture of a reaction coordinate with a change in energy for a particular reaction, it starts out at high energy goes up higher and then comes down to a lower energy. It identifies four regions of this curve one, 2, 3, and four. And it wants you to identify what is each particular number For reaction coordinate such as this ...
Stretchable broadband photo-sensor sheets for nonsampling, … 11.05.2022 · While device design on the stretchable PTE sensor sheet has promoted the BBR-based passive photo-monitoring techniques as was intended, further efforts on the following topics potentially accelerate developing the scheme presented here. Wireless device driving is one of them (fig. S28), for example. Figure S29 demonstrates the wireless PTE signal readout …
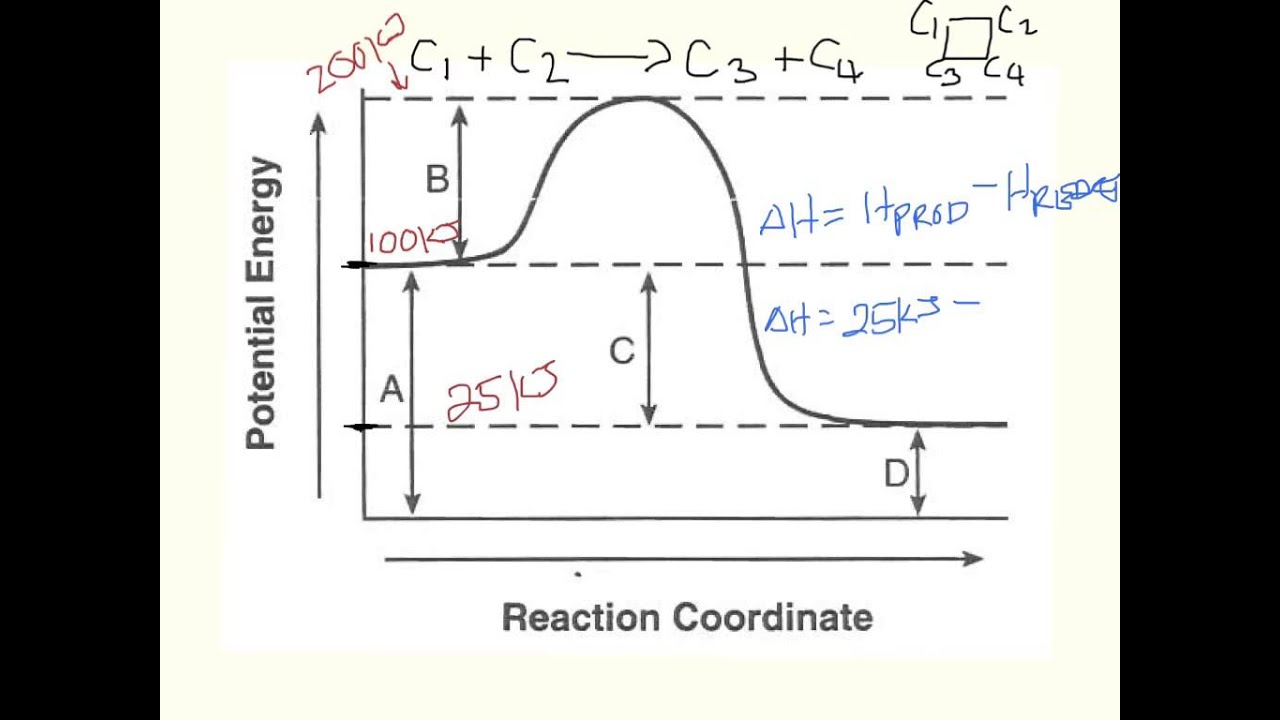
How to draw the potential energy diagram for this reaction? 2. Identify the sign of the enthalpy change, ΔH, along with its value. The decrease in chemical potential energy should be the same as the amount of thermal energy released. That is. Ereactants − Eproducts = 2219.9lkJ⋅ mol−1. However, since. ΔH = Eproducts −Ereactants , ΔH = −2219.9lkJ⋅ mol−1. Note that by convention, the ...
Endothermic Reaction Coordinate Diagram - schematron.org The fully filled in reaction coordinate diagram is displayed below. This reaction is also exothermic because the energy of the products is lower than that of the. In the case of an endothermic reaction, the reactants are at a lower energy level compared to the products—as shown in the energy diagram below. In other.Start studying CHEMISTRY 2.


Post a Comment for "42 label the following reaction coordinate diagram."